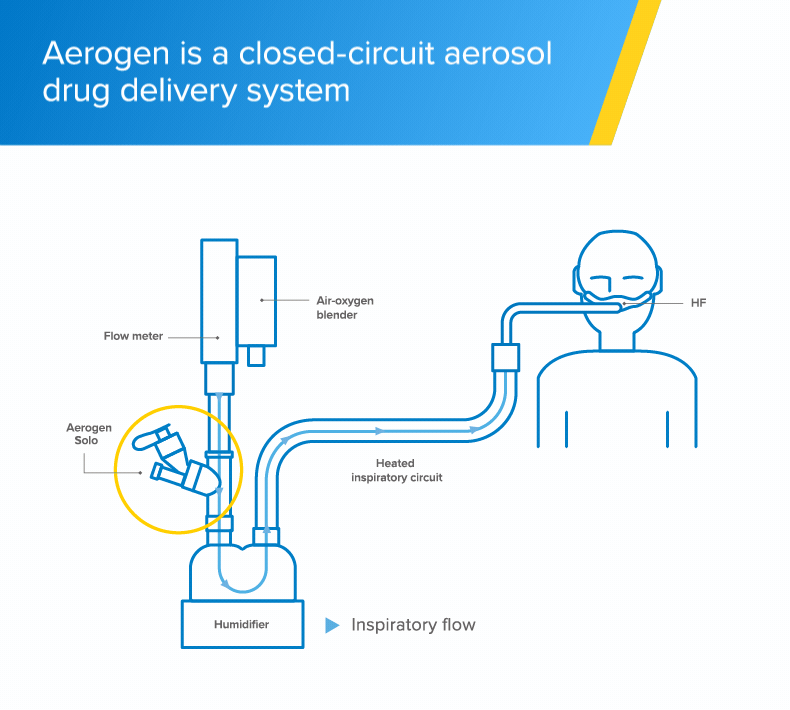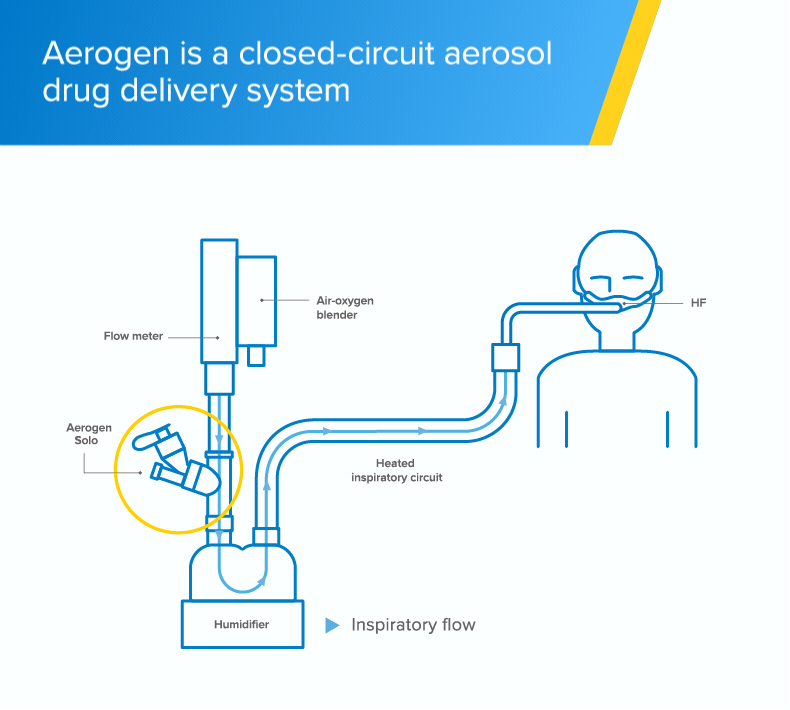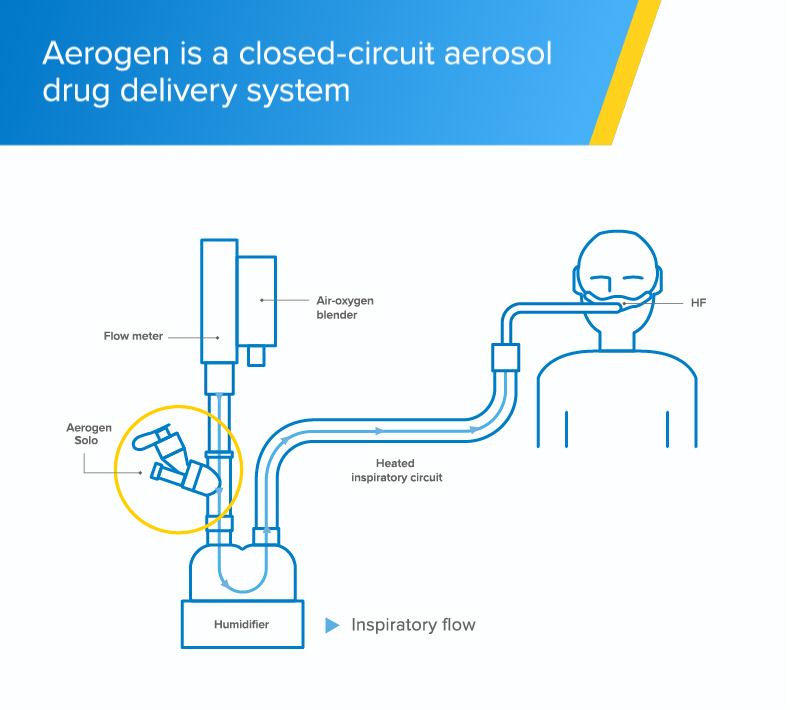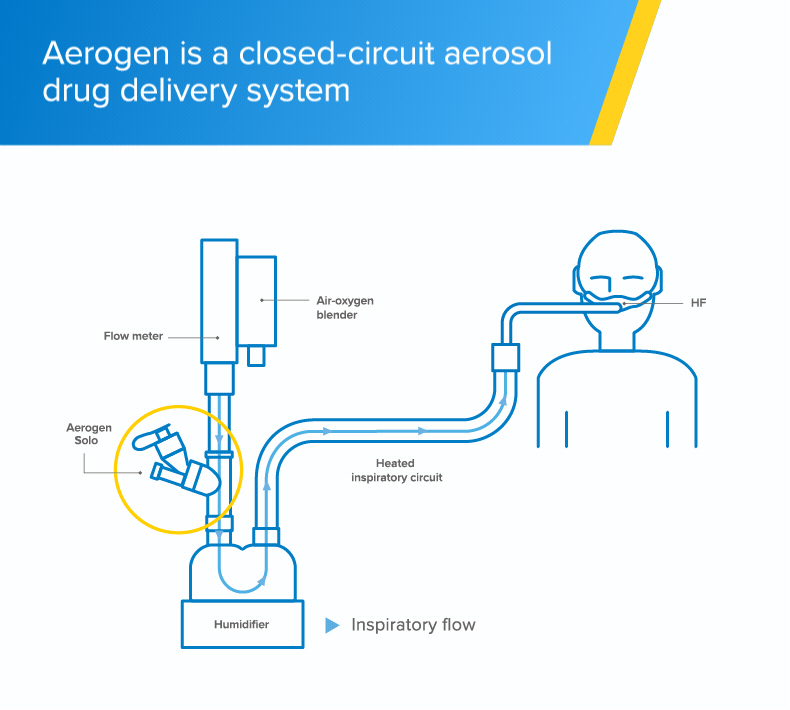
High-flow
The Aerogen Solo vibrating mesh nebuliser delivers aerosol medication to your patients during high-flow therapy1
Integrated aerosol delivery
The Aerogen Solo vibrating mesh nebuliser delivers integrated aerosol delivery with high-flow1,2
- The Aerogen Solo - a vibrating mesh nebuliser - fits in-line with no added flow and no interruption of therapy during administration of medication1
- With Aerogen, the circuit can be maintained during aerosol therapy1 unlike conventional nebulisation methods that require interruption of high-flow to use a facemask or mouthpiece†2
†Survey of worldwide clinical practice of HF and concomitant aerosol therapy in the adult ICU setting
Ease of use
The Aerogen Solo vibrating mesh nebuliser simplifies the workflow1,2
- In studies, in-line aerosol drug delivery has been associated with better comfort*2,†3,‡4 and improved convenience versus conventional aerosol therapy*2
- One system can be used throughout a patient's respiratory journey (IMV, NIV, HF, SV),1 supporting continuity of care
*Survey of worldwide clinical practice of HF and concomitant aerosol therapy in the adult ICU setting. Conventional aerosol therapy consisted of a vibrating mesh nebuliser, ultrasonic nebuliser or jet nebuliser used with a facemask
†Please refer to 30-354 REV U Aerogen Solo Instruction Manual when using Aerogen
‡A randomised, cross-over study in infants with bronchiolitis comparing in-line Aerogen vs jet nebuliser with a facemask
Using Aerogen†

Effectiveness
Aerogen provides effective medication delivery during high-flow therapy5–10
In studies, in-line aerosol drug delivery with Aerogen during high-flow was associated with:
- ~4x more medication delivered to the lungs (3.6%) versus a jet nebuliser (1.0%)†5
- 3.5% –17% medication delivery to the lungs, depending on flow rates†6
Aerogen supports bronchodilator response during high-flow7–10
In studies of patients with COPD or asthma, in-line aerosol drug delivery with Aerogen during high-flow has been associated with:
- Significant improvements in lung function (FEV1, FVC and PEF) following salbutamol nebulisation via high-flow versus high-flow alone in patients with severe exacerbation of COPD9
- Effective bronchodilator response, even with a gas flow of 50 L/min‡10
†Study performed in healthy subjects
‡At cumulative doses of 1.5–3.5 mg salbutamol in patients with mild-to-moderate COPD or asthma
COPD, chronic obstructive pulmonary disease; FEV1 forced expiratory volume in 1 second; FVC, forced vital capacity; PEF, peak expiratory flow

Representative scintigraphy images of pulmonary deposition across different flow rates at 10, 30, and 50 L/min in healthy adults.†6
Guidelines
Clinical and scientific societies around the world recommend the use of closed-circuit nebulisers for the management of patients with COVID-19 requiring aerosol drug delivery:
- AARC: Guidance 202011
- ISAM: Interim Guidance 202012
- Spanish Scientific Societies: Expert Clinical Consensus 202013
- Chinese Thoracic Society: Respiratory Care Committee 202014
- Indian Society of Critical Care Medicine: Position Statement 202015
- Indian Chest Society: Guidance 202016
Globally renowned
Aerogen technology has been in use for over 25 years, in more than 80 countries globally and is associated with over 300 clinical papers and publications.17 Aerogen is the partner of choice for the world's leading ventilator companies for aerosol drug delivery.17







Enquiries
The Aerogen team and our representatives are available globally to answer your questions, provide an online demonstration and place orders
- Aerogen Solo Instruction Manual
- Li J, Tu M, Yang L, et al. Respir Care. 2021;66(9):1416-1424.
- Valencia-Ramos J, Miras A, Cilla A, et al. Respir Care 2018;63(7):886–893.
- Valencia-Ramos J, Ochoa Sangrador C, García M, et al. Arch Dis Child. 2022;archdischild-2021-323161.
- Dugernier J, Hesse M, Jumetz T, et al. J Aerosol Med Pulm Drug Deliv. 2017;30(5):349-358.
- Alcoforado L, Ari A, Barcelar JM, et al. Pharmaceutics. 2019;11(7):320.
- Reminiac F, Vecellio L, Bodet-Contentin L, et al. Ann Intensive Care. 2018;8(1):128.
- Li J, Zhao M, Hadeer M, Luo J, Fink JB. Respiration. 2019;98(5):401-409.
- Beuvon C, Coudroy R, Bardin J, et al. Respir Care. 2021;respcare.09242.
- Li J, Chen Y, Ehrmann S, Wu J, Xie L, Fink JB. Pharmaceutics. 2021;13(10):1655.
- American Association for Respiratory Care SARS CoV-2 Guidance Document. https://www.aarc.org/wp-content/
- Fink JB, Ehrmann S, Li J, et al. J Aerosol Med Pulm Drug Deliv. 2020;33(6):300-304.
- Cinesi Gómez C, Peñuelas Rodríguez Ó, Luján Torné M, et al. Med Intensiva (Engl Ed). 2020;44(7):429-438.
- Respiratory care committee of Chinese Thoracic Society. Zhonghua Jie He He Hu Xi Za Zhi. 2020;17(0):E020.
- Kumar S, Mehta S, Sarangdhar N, et al. Expert Rev Respir Med. 2021;15(4):519-535.
- Swarnakar R, Gupta NM, Halder I, et al. Lung India. 2021;38(Supplement):S86-S91.
- Aerogen Data on File.
GL-3462-1-EN