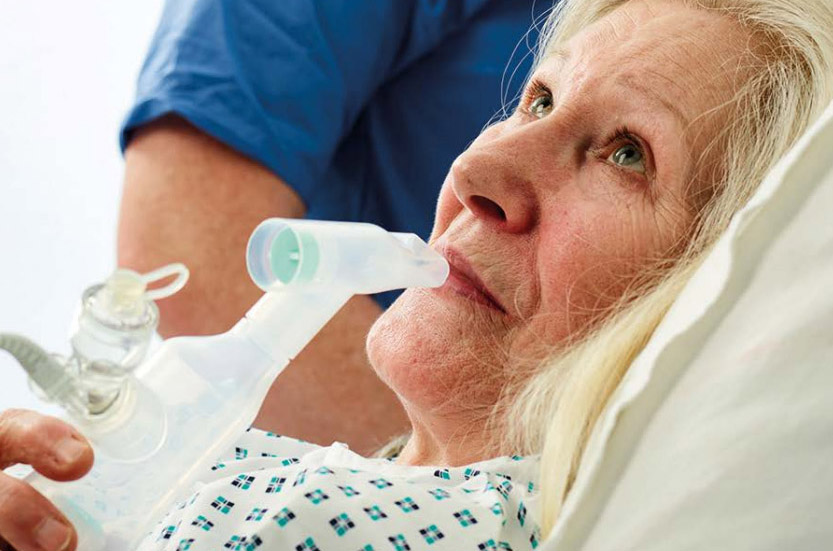
Spontaneous breathers
Aerogen vibrating mesh nebulisers deliver aerosol medication to your self-ventilating patients requiring symptom control for respiratory exacerbations1,2
Aerosol drug delivery
Aerogen vibrating mesh nebulisers provide effective medication delivery during self-ventilation1–4
In studies, bronchodilator administration via the Aerogen Ultra was associated with:
- 6x more medication delivered to the lungs during self ventilation versus a jet nebuliser†3
- Significantly less medication left in the cup versus other nebulisers‡4
†Study performed in healthy subjects; between-group difference: 34.1% vs 5.2%; p<0.001
‡In-vitro/ex vivo models
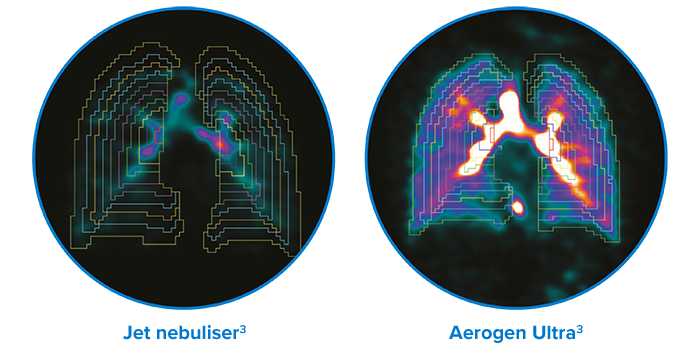
Representative scintigraphy images of pulmonary aerosol deposition in healthy adults
Ease of use
Aerogen vibrating mesh nebulisers simplify the workflow
- Virtually silent drug delivery,5 keeping a calm environment for your patients
- No added flow required5
- Aerogen Solo - a vibrating mesh nebuliser - is intended for the aerosolisation of any physician prescribed medications for inhalation which are approved for use with a general purpose nebuliser5
- One system used throughout a patient's respiratory journey (IMV, NIV, HF, SV),5 supporting continuity of care
Effectiveness
Aerogen vibrating mesh nebulisers improve patient care in response to aerosolised medication1,2
In studies, when compared with jet nebulisers, bronchodilator administration via Aerogen Ultra was associated with:
- Significantly fewer treatments and significantly less time required to achieve symptom control†1
Across all patients in an emergency department :
- ~30% relative reduction in hospital admission rates2
- 85% of patients achieving symptom control with one 2.5 mg salbutamol dose2
- 37-minute reduction in ED median length of stay2
†Defined as achieving a mild asthma score following an asthma exacerbation
Guidelines
Clinical and scientific societies around the world recommend the use of closed-circuit nebulisers for the management of patients with COVID-19 requiring aerosol drug delivery:
- AARC: Guidance 20206
- ISAM: Interim Guidance 20207
- Spanish Scientific Societies: Expert Clinical Consensus 20208
- Chinese Thoracic Society: Respiratory Care Committee 20209
- Indian Society of Critical Care Medicine: Position Statement 202010
- Indian Chest Society: Guidance 202011
Globally renowned
Aerogen technology has been in use for over 25 years, in more than 80 countries globally and is associated with over 300 clinical papers and publications.12 Aerogen is the partner of choice for the world's leading ventilator companies for aerosol drug delivery.12







Enquiries
The Aerogen team and our representatives are available globally to answer your questions, provide an online demonstration and place orders
- Moody GB, Luckett PM, Shockley CM, et al. Respir Care 2020;65(10):1451–1463.
- Dunne RB, Shortt S. Am J Emerg Med. 2018;36(4):641-646.
- Dugernier J, Hesse M, Vanbever R, et al. Pharm Res. 2017;34(2):290-30.
- Lin H-L, Fang T–P, Cho H-S, et al. Pulm Pharmacol Ther. 2018;48:225-231.
- Aerogen Solo Instruction Manual
- American Association for Respiratory Care SARS CoV-2 Guidance Document. https://www.aarc.org/wp-content/
- Fink JB, Ehrmann S, Li J, et al. J Aerosol Med Pulm Drug Deliv. 2020;33(6):300-304.
- Cinesi Gómez C, Peñuelas Rodríguez Ó, Luján Torné M, et al. Med Intensiva (Engl Ed). 2020;44(7):429-438.
- Respiratory care committee of Chinese Thoracic Society. Zhonghua Jie He He Hu Xi Za Zhi. 2020;17(0):E020.
- Kumar S, Mehta S, Sarangdhar N, et al. Expert Rev Respir Med. 2021;15(4):519-535.
- Swarnakar R, Gupta NM, Halder I, et al. Lung India. 2021;38(Supplement):S86-S91.
- Aerogen Data on File
GL-3463-1-EN